Ants, jellyfish and ground beetles use it to defend themselves and when hunting. How effectively it can protect stinging nettles against predators is something many of us have experienced first hand: the substance in question is formic acid. The acrid and pungent smelling liquid produced by nature already awakened people's curiosity many centuries ago. The English naturalist John Ray first isolated the simplest of the carboxylic acids in 1671. He heated red wood ant bodies in a glass flask and obtained in the distillate an acidic liquid he called formic acid. The first successful chemical synthesis was achieved by the French chemist Marcellin Berthelot in 1855. BASF's interest in formic acid began in the 1920s. When an increasing number of industry sectors started using carboxylic acid, the chemical company launched large scale production in 1935. Today, there are hardly any limits to the use of formic acid. "It's a real all-rounder," emphasizes Dr. Tatjana Levy, Innovation Manager in BASF's Intermediates division. The acid has a history of successful use in numerous applications over several decades. In animal nutrition it preserves feeds, it is used in leather and textile manufacture and is a constituent of oilfield chemicals. "And we are constantly discovering new applications that we are developing together with our customers," adds Levy.
Airports place high demands on deicing agents
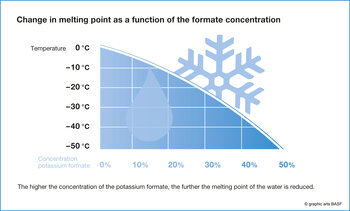
European airports are also among the users. "They have been using the salts of formic acid for more than ten years to deice their landing strips and taxiways," explains Levy. The salts, also known as formates, make sure that water doesn't freeze at zero degrees Celsius as it usually does. Depending on the concentration of the deicing agent, the freezing point is reduced to as much as minus 50 degrees Celsius and is then much lower than the outdoor temperature. In this way, formates rapidly remove thin layers of ice and effectively prevent new snow from sticking to the landing strip or a new layer of ice from forming. Formates are also very eco-friendly. "If the salts of formic acid enter the airport waste water treatment plant together with the melt water, their waste water polluting effect is much less than that of other deicing agents because of their good biodegradability and low oxygen demand during the breakdown process," says Levy.
The snow clearing service of Zurich airport has been using formates since 2005. "We place particularly high demands on deicing agents for safety and environmental protection reasons," emphasizes Hanspeter Moll, Head of Airfield Maintenance Operations at Zurich airport. "They must deice runways and taxiways rapidly and lastingly, have good material compatibility and also be eco-friendly. Our experience has shown that the salts of formic acid meet these requirements better than all other deicing agents."
Local authorities increasingly aware of formate benefits
The positive experiences of airports have made local and municipal authorities increasingly aware of formates as an alternative deicing agent. Snow clearing services in Scandinavia, Switzerland and Austria use the organic salt to deice their roads, bikeways and sidewalks in sensitive areas such as boulevards with mature tree stocks or in districts with many historic buildings. The City of Basel has been using the thawing agent for many years to remove residual snow from its artificial turf areas. "We first clear the snow and ice mechanically," explains Eric Hardman, responsible for sports facilities at Basel City Sports Office. Formates are then applied to melt the remaining thin layer of snow. Their good deicing performance makes sure the sports ground is ready for use again in a short time. "We were also impressed by the fact that the salts of formic acid are also biodegradable at low temperatures and do not affect the athletes. Moreover, artificial turf grounds and sports utensils like balls, rackets, goalposts or nets survive the cold season undamaged when formates are used as deicing agents," says Hardman.
Although winter clearance using formates is more expensive than with ice-melting salt or slippage preventing agents like grit or sand, the procurement costs are not so high when the follow-on costs of the various deicing agents are factored in. Sodium chloride-based ice melting salt disturbs the water and nutrient balance of plants in the soil. Its corrosive effect also damages buildings, roads and bridges. The effectiveness of slippage preventing agents is controversial, as their use increases particulate pollution in cities and its cleanup is laborious. The salts of formic acid, on the other hand, are eco-friendly, hardly corrosive and reliably keep roads and walkways free from ice and snow – without undesirable side effects. Replanting of trees or shrubs or expensive repairs to buildings after the winter are no longer necessary.
Nature is and remains the largest producer
BASF is one of the world's leading manufacturers of formic acid. Whereas in Asia, South America and Southern Europe the majority of the acid is used for leather processing, in Central and Northern Europe the main purchasers are agriculture and the animal feed industry. BASF operates production plants for formic acid in Ludwigshafen, Germany and Nanjing, China. Another facility is planned to come on stream in 2014 at the Geismar site in the US Federal State Louisiana. The company will then have increased its annual capacity to more than 300,000 metric tons.
But this still doesn't make BASF the largest producer of formic acid. This is and remains the privilege of the countless animals and plants which produce more formic acid annually than all the factories in the chemical industry put together – and have been doing so for many millions of years.
Technology
Ice-free through the winter
Kopiert!